What is the difference between 'ionic bonding' and 'covalent bonding'?
1 Answer
This is an old story, and I apologize if I rabbit on about nothing to the power of less...(so what's new here?).
Explanation:
The modern covalent bond is conceived to be a region of high electron density between two positively-charged atomic nuclei such that nucleus-nucleus repulsion is negated and a NET ATTRACTIVE force operates between atomic nuclei and the electron cloud.
And we can map electron density to illlustrate the electronic density...
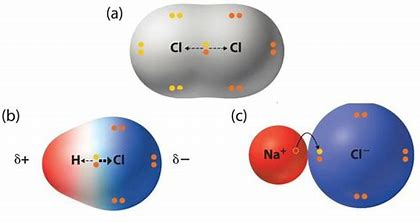
And so there is substantial electronic density BETWEEN the positively charged atomic nuclei, which allows the CLOSE approach of such nuclei. Now covalent bonding can give rise to discrete molecules, and also to non-molecular materials that feature atom/atom interaction across the entire matrix. And so we say that covalent bonding results from the sharing of electrons.
On the other hand, for ionic bonding, there is ELECTRONIC TRANSFER from the metal, to give a small cation, to a non-metal anion. And the result is that the cation is SMALLER than the parent metal atom, and the ANION is larger than the parent non-metal atom. Necessarily there is electrostatic attraction between positive and negative ions, which follows Coulomb's old inverse square law. In the ionic lattice, which features interpenetrating arrays of cations, and anions, there is a regular ordered array, and the smallest neutral anion/cation combination gives rise to the ionic formula.
Of course in the ionic lattice there is electrostatic REPULSION between like-charged ANIONS, and like-charged CATIONS.. But there is also ELECTROSTATIC ATTRACTION between CATIONS and ANIONS all over the lattice. And if you sum up all the attractive versus repulsive interactions across the entire ionic lattice, A NET ATTRACTIVE FORCE RESULTS (and this can certainly be done quantitatively).... And thus ionic bonding results from the transfer of electrons between atoms...

