What is the empirical formula of a compound that is 26.58% K, 35.35% Cr, and 38.07% O?
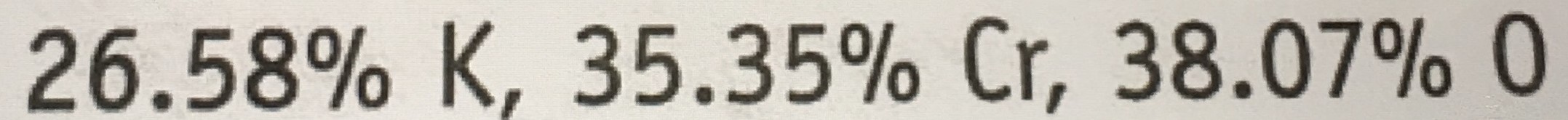
1 Answer
The empirical formula is
Explanation:
The empirical formula represents the lowest whole number ratios of elements in a compound. You must first determine the number of moles of each element, then determine the mole ratios. The mole ratios, or a multiple of the mole ratios, will be the subscripts for the elements in the empirical formula.
Determine moles.
Since the percentages add up to
Divide the given number of grams for each element by its molar mass (atomic weight on the periodic table in grams/mole). Since molar mass
Determine mole ratios.
The mole ratios are determined by dividing the number of moles for each element by the least number of moles. If they all come out to whole numbers, then the mole ratios are the subscripts for the empirical formula. If they do not come out to whole numbers, they must be multiplied by a number that will result in whole number.
Since the mole ratio for oxygen is
Empirical Formula:
This represents the formula unit for the ionic compound potassium dichromate.

