What is the half-life of a radioactive isotope if a 500.0 g sample decays to 62.5 g in 24.3 hours?
2 Answers
Aug 18, 2017
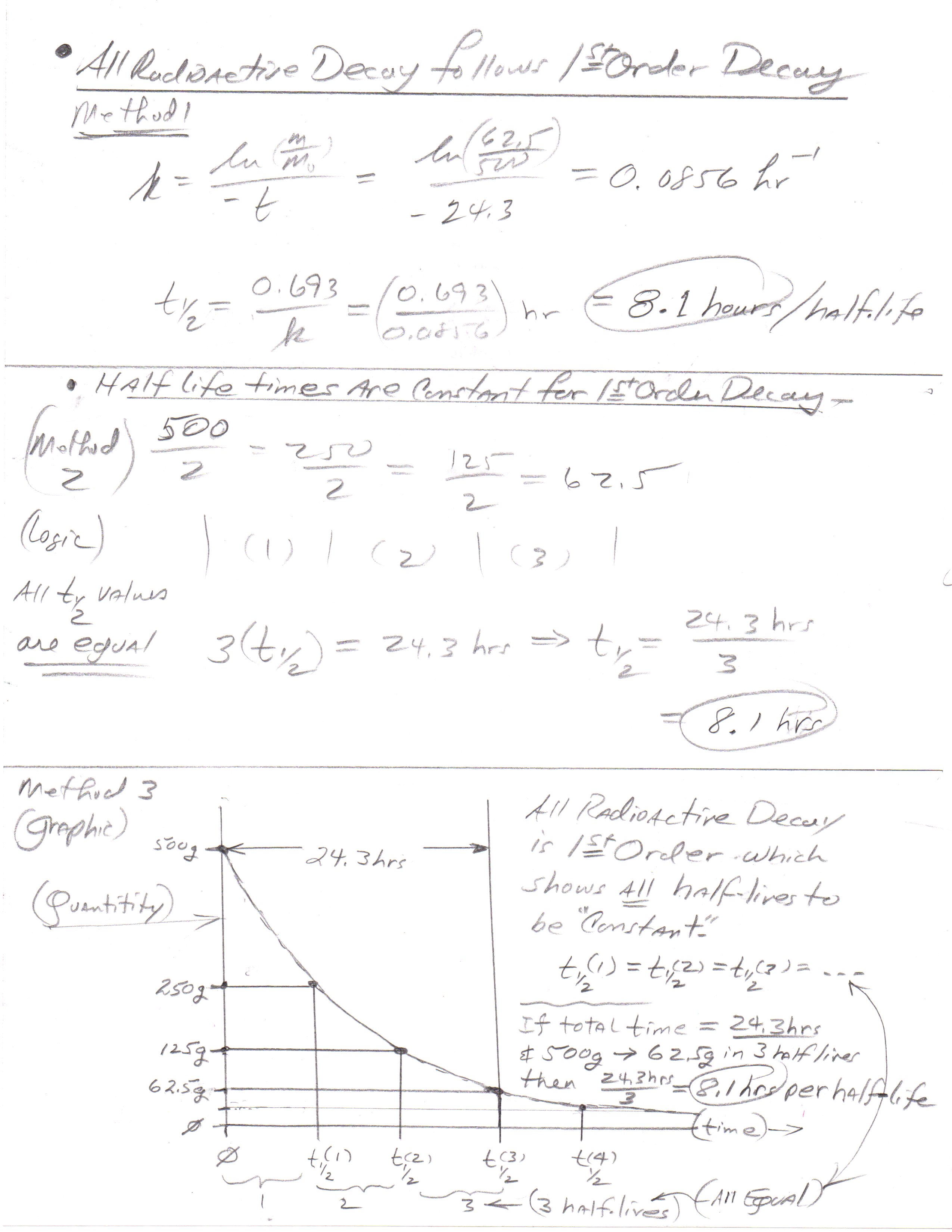
8.10 hours.
Explanation:
You start with 500.0g.
After the first half-life, you have 250.0g.
After the second, you have 125.0g.
After the third, you have 62.50g.
Therefore, it takes three half-lives to decay to 62.50g.
Therefore, the elapsed time must be triple the length of one half-life.
Aug 18, 2017
Explanation:
All radioisotope decay follows 1st order kinetics and shows that the half-live value is constant from one half life interval to another. The following shows three approaches to solving the question posted.
x