What is the name of this alkane?
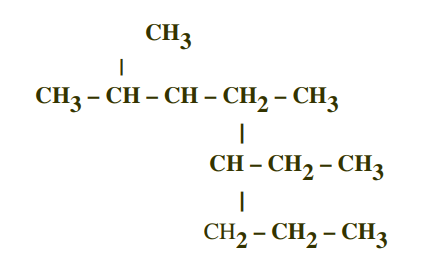
I got 5-ethyl-2,4-dimethyloctane but the answer came up as this
I got 5-ethyl-2,4-dimethyloctane but the answer came up as this
2 Answers
You are missing a hydrogen in the line formula......
Explanation:
And also you have numbered the ethyl group at
So my attempt to muddy the waters is......
Is the molecule saturated? Would you expect it to be solid, liquid, or gas under standard conditions?
Here's what I get.
Explanation:
Are you trying to name this compound?
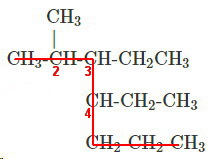
Step 1. Find the longest continuous chain of carbon atoms
The longest continuous chain contains seven carbon atoms, so the main chain is heptane.
Step 2. Number from the end closest to a substituent
There are substituents at carbons 2, 3, and 4.
Step 3. Name the substituents
They are methyl, ethyl, and ethyl.
Step 4. Name the compound
List the substituents in alphabetical order (
Use numbers to locate the substituents, and use hyphens to join letters and numbers (no spaces!).
The name is 3,4-diethyl-2-methylheptane.


