The rate law is
#r = k"[A]"^m#
where
#rcolor(white)(l) =# the reaction rate
#k color(white)(l)=# the rate constant
#m =# an integer whose value we must determine
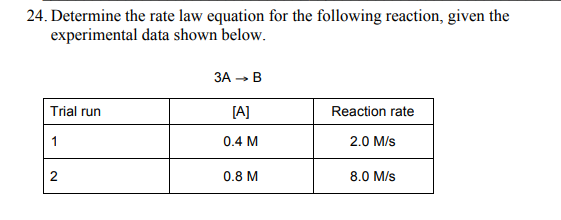
Consider Trial Runs 1 and 2
#r_2/r_1 = (color(red)(cancel(color(black)(k)))(0.8 color(red)(cancel(color(black)("mol/L")))) ^m)/(color(red)(cancel(color(black)(k)))(0.4 color(red)(cancel(color(black)("mol/L"))))^m) = (0.8/0.4)^m = (8.0 color(red)(cancel(color(black)("mol·L"^"-1""s"^"-1"))))/ (2.0 color(red)(cancel(color(black)("mol·L"^"-1""s"^"-1"))))#
#2^m =4.0#
Thus, doubling the concentration quadruples the rate.
The reaction is second order in #["A"]#.
If you use logarithms, you get
#color(blue)(mlog2 = log4.0)#
#color(blue)(m = log4.0/log2 = 2.0 ≈ 2)#
The reaction is second order in #"[A]"#.
The rate law is #r = k["A"]^2#.