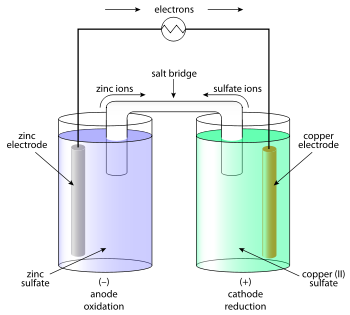
What substance is reduced in the following redox reaction?

1 Answer
Mar 21, 2018
Well,
Explanation:
A species undergoes reduction when its oxidation number is
and so...
i.e.
The electrons are proposed to come from somewhere, i.e. from zinc metal, the which is conceived to LOSE electrons, and is
And to write the electrochemical cell, we simply add the individual redox equations in such a way that the electrons, virtual particles of convenience are eliminated...
...to give finally...
This is the basis of the original galvanic cell....