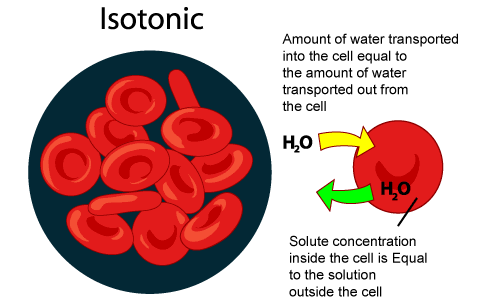
When a cell is in iso tonic solution water moves in and out of the cell. There's no net movement though. Am i correct?
1 Answer
Yeah! You are correct.
Explanation:
Because isotonic solution means having same osmotic pressure. So, if we place a cell in an isotonic solution so it means: we are placing a cell in a solution which is quite similar to cell's solution.
So there will be no net movement of molecules across the membrane and there will also not any kinda net change in the concentration of solvents and solutes on both sides of membrane. Any kind of diffusion or osmosis will not take place because the equilibrium has been achieved. There will only be free movement of water across the membrane without changing the concentration of solutes on either side, in case of isotonic environment.
http://biologyform4.blogspot.com/2013/09/hypotonic-hypertonic-and-isotonic_8747.html
Hope it helps...

