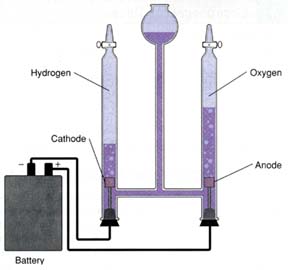
When water is electrolyzed, hydrogen and oxygen are produced in the ratio 2:1. Why?
1 Answer
The mole ratio of hydrogen to oxygen in the water molecule,
Explanation:
The chemical equation of the synthesis of water is:
We can see from the balanced equation, that the mole ratio of hydrogen to oxygen is
The electrolysis of water causes the decomposition of water,
The balanced equation is:
The mole ratio of hydrogen to oxygen is