Which of the molecules below has the highest boiling point?
Explain your choice in 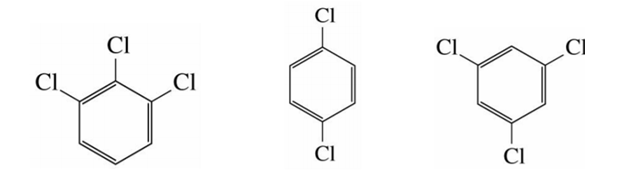 terms of intermolecular forces, listing all types of intermolecular forces involved
terms of intermolecular forces, listing all types of intermolecular forces involved
Explain your choice in  terms of intermolecular forces, listing all types of intermolecular forces involved
terms of intermolecular forces, listing all types of intermolecular forces involved
1 Answer
I would assume the
Explanation:
The trichloro derivatives should be more involatile than the dichloro derivative, in that intermolecular force, as the dispersion force, INCREASE with the number of electrons.
And so now we got to compare
But as chemists, as physical scientists, we ARE not done. We have made a reasoned decision...please look up the melting points and boiling points of these materials to see that we are not mistaken (for once!)... My Aldrich catalogues says that the boiling point of
Note that you are NOT expected to know these data....YOU ARE EXPECTED to interpret them....

