Why higher energy is required for stretching vibration as compared to the bending vibrantion?
1 Answer
Mar 8, 2018
Here's what I get.
Explanation:
Stretching vibrations
In a stretching vibration, the atoms move in and out along the internuclear axis.
The nuclei are moving apart against the attraction of the bonding electrons between them.
Bending vibrations
In a bending vibration, the bond length does not change.
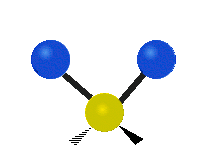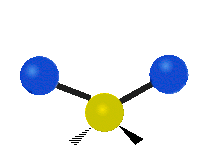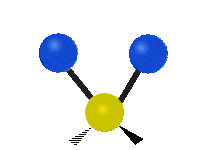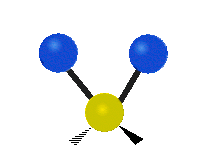
Instead, the bond angle changes.
The angle distorts from the ideal angle for the orbitals (say, 120° or 109.5°).
It is easier to bend a bond than to stretch it because the nuclei are not moving against the attraction of the bonding electrons.
Thus, a stretching vibration has a higher frequency and requires more energy than a bending vibration.

