Why is it important that we know the initial concentration of NaOH very accurately and yet for crystal violet, we do not need to know its initial concentration? (We aren't using volumetric glassware when we add 3 drops of crystal violet)
1 Answer
Mar 22, 2018
Are you asking why we DON'T need to know the concentration of the indicator?
Explanation:
We titrate the base with stoichiometric acid...or so I think....and the best way of determining the end-point is to add a FEW drops of indicator, the which usually change colour PRECISELY at the endpoint...(i.e. within 1-2 drops, i.e. approx.
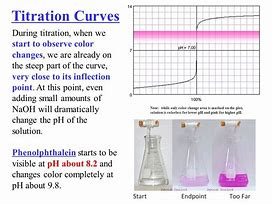
The graph shows the PRECIPITOUS rise in

