Why methanol has a three fold symmetry ?
In Newman projection, it seems methanol should have 2 fold symmetry, but methanol has a three fold symmetry. Why it is so.
In Newman projection, it seems methanol should have 2 fold symmetry, but methanol has a three fold symmetry. Why it is so.
1 Answer
Dec 13, 2017
Are you sure....?
Explanation:
This is really a scenario where it is advizable to construct a model of the molecule in question....
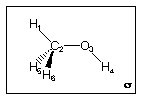
As written, the molecule has

