Would someone please help me? When doing isomers of pentene, how do you know that you have 6?
For instance, for Pentene, it says to write all six condensed structural isomers? I mean, how do you know you have only 6? I have a professor that doesn't know how to get the information out to his students and it's very frustrating. please help. THANK YOU SOO VERY MUCH!!
For instance, for Pentene, it says to write all six condensed structural isomers? I mean, how do you know you have only 6? I have a professor that doesn't know how to get the information out to his students and it's very frustrating. please help. THANK YOU SOO VERY MUCH!!
2 Answers
You only know if you already have done it... Pentene implies
Having a ring adds another degree of unsaturation, so it must also be straight-chained so that the degree of unsaturation is
Here's what I got:

-
I started from the straight-chained alkene and moved the
#pi# bond until I reached the reflection plane that bisects the molecule. That's#2# . Then I looked at any cis/trans isomers to get a total of#3# so far. -
Then I moved the
#"C"-"C"# bond to start branching. I can only move it one over, as there is a reflection plane bisecting the resultant butene. From there, I moved the#pi# bond around: on the end, in the middle, and on the other end. That gives#3# more.
You can convince yourself that moving
Warning! Long Answer. Here's how I would do it.
Explanation:
There are more than six isomers of pentene, but your professor apparently wants only the alkene isomers of pentene.
You must approach the problem systematically. Then you continue until you can find no more isomers.
Step 1. Start with five carbon atoms in a row
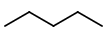
Step 2. Insert a
You get

and
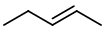
Note that pent-2-ene exists as cis and trans isomers.
The isomer above is trans-pent-2-ene.
cis-Pent-2-ene has the structure below.
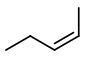
Step 3. Now, start with a four-carbon chain and a methyl group in all possible positions
The is only one possible structure:
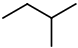
Again, insert a
You get three more isomers:
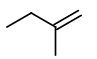
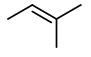
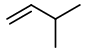
Step 4. Now, start with a three-carbon chain and two methyl group in all possible positions
Only one new structure is possible.
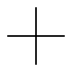
Insert a
We can't do it! Any place we try to insert a
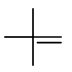
Here we stop.
We have generated six alkene isomers. There are no more.


