Double Replacement Reactions
Key Questions
-
Answer:
Balance the ions as groups ...
Explanation:
Double replacement reaction occurs when the cations and anions of two ionic compounds are exchanged. The figure below clearly illustrates how this swap takes place.
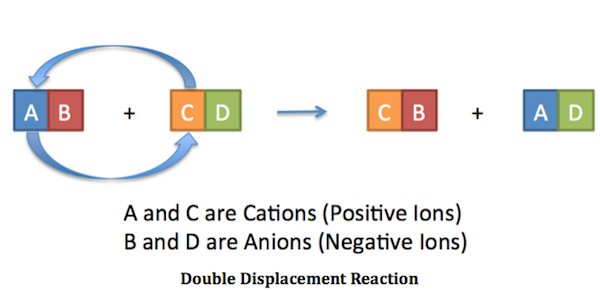
When the chemical formula for each ionic compounds is written correctly, you can balance the equation like any other chemical equations by making sure the number of atoms for each element is the same on the left and the right.
If you are interested in a "simplified" way of balancing double replacement reaction, you can balance "A", "B", "C" and "D", meaning, handling the cations and anions as a group, rather than as individual elements.
To illustrate, here's an example of a double displacement reaction:
#NaOH + FeCl_2 -> Fe(OH)_2 + NaCl# The groups of ions are:
A = Na
B = OH
C = Fe
D = ClNow, we'll count the number of each groups on both sides:
Left - Group - Right
1 - A (Na) - 1
1 - B (OH) - 2
1 - C (Fe) - 1
2 - D (Cl) - 1Looks like OH and Cl are not balanced. We'll need to place a
#color(red)2# in front of NaOH (on the left) and#color(red)2# in front of NaCl (on the right).#color(red) 2 NaOH + FeCl_2 -> Fe(OH)_2 + color(red) 2 NaCl# Including the physical states:
#2 NaOH"(aq)" + FeCl_2"(aq)" -> Fe(OH)_2"(s)" + 2 NaCl"(aq)"# -
A double displacement precipitation is a reaction in which two soluble ionic compounds react to form an insoluble precipitate.
Double replacement reactions take the general form:
A⁺B⁻ + C⁺D⁻ → A⁺D⁻ + C⁺B⁻
Example
AgNO₃(aq) + NaCl(aq) → AgCl(s) + NaNO₃(aq)
Ag⁺(aq) + NO₃⁻(aq) + Na⁺(aq) + Cl⁻(aq) → AgCl(s) + Na⁺(aq) + NO₃⁻(aq)
This is a double replacement reaction, because the silver ion and the sodium ion have exchanged partners. It is also a precipitation reaction, because the silver ion and the chloride ion are removed from the solution as solid silver chloride.
Here is a video which shows another example of a double replacement (precipitation) reaction.
Detailed and organized can be on this site http://homepage.smc.edu/walker_muriel/double_displacement_reactions_procedure.htm