Density
Key Questions
-
Answer:
They are related by the the density triangle.
Explanation:
They are related by the the density triangle.
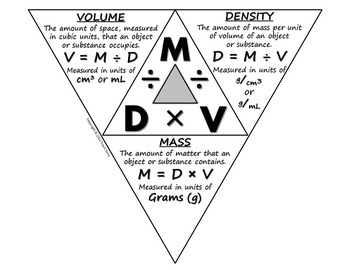
d =
m/VmV m = d×V
V =
m/dmd DENSITY
Density is defined as mass per unit volume.
d =
m/VmV Example:
A brick of salt measuring 10.0 cm x 10.0 cm x 2.00 cm has a mass of 433 g. What is its density?Step 1: Calculate the volume
V = lwh = 10.0 cm × 10.0 cm × 2.00 cm = 200 cm³Step 2: Calculate the density
d =
m/VmV =(433 g)/(200 cm³) = 2.16 g/cm³MASS
d =
m/V We can rearrange this to get the expression for the mass.
m = d×V
Example:
If 500 mL of a liquid has a density of 1.11 g/mL, what is its mass?m = d×V = 500 mL ×
(1.11 g)/(1 mL) = 555 gVOLUME
d =
m/V We can rearrange this to get the expression for the volume.
V =
m/d Example:
What is the volume of a bar of gold that has a mass of 14.83 kg. The density of gold is 19.32 g/cm³.Step 1: Convert kilograms to grams.
14.83 kg ×
(1000 g)/(1 kg) = 14 830 gStep 2: Calculate the volume.
V =
m/d = 14 830 g ×(1 cm³)/(19.32 g) = 767.6 cm³ -
You can identify an unknown substance by measuring its density and comparing your result to a list of known densities.
Density = mass/volume. Assume that you have to identify an unknown metal. You can determine the mass of the metal on a scale. You can determine the volume by dropping the object into a graduated cylinder containing a known volume of water and measuring the new volume. You divide the mass by the volume and compare the density to a list of known densities.
EXAMPLE
A metal bolt with a mass of 99.7 g is dropped into a graduated cylinder containing 50.0 cm³ of water. The new volume reads
72.1 cm³. Identify the metal.Solution
V = 72.1 cm³ - 50.0 cm³ = 22.1 cm³
D =
m/V = (99.7 g)/(22.1 cm³) = 4.51 g/cm³Now you compare your density with a list obtained from your instructor or from an on-line source such as
https://sites.google.com/site/chempendix/densities-of-pure-metals
What metal was your bolt made of?
-
Answer:
Density is the mass per unit of volume of a substance.
Explanation:
Density measures the compactness in molecular arrangement in any substance which determines how heavy or light any substance is.
The density formula is
"density" ="mass"/"volume" . Mass units are most commonly grams or kilograms. Volume units are most commonly cubic centimeters ("cm"^3 ), cubic meters ("m"^3 ), or millileters (mL).Examples of density include the following:
The density of water at
"4"^"o""C" can be written as"1.000g/cm"^3 ,"1.000g/mL" ,"1000Kg/m"^3 , and"1.000kg/L" .The density of iron at
"0"^"o""C" is"7.874g/cm"^3 and"7874kg/m"^3 .The density of sodium metal at
"0"^"o""C" is"0.968g/cm"^3 , and"968kg/m"^3 .In order to determine the density of a substance, you need to know its mass and its volume. Then divide its mass by its volume, remembering to divide the units as well.
Example
A"1.26cm"^3 sample of the element mercury has a mass of"17.05g" . What is it's density?Solution
"density" ="mass"/"volume" ="17.05g"/"1.26cm"^3 ="13.5g/cm"^3" Here is a video example of how to solve a density problem.