Bond Line Notation
Key Questions
-
Perhaps the first question one would ask when curious about organic chemistry!
An ethane molecule
H3C−CH3 is merely,
Each terminal end has three hydrogens! It's that easy!
However, when we connect these lines into a chain or into a ring is when things may seem confusing. Consider propane and cyclopropane, respectively,

Where each line connects is a
CH2 group.If there are substituents anywhere, you'd merely understand that it has one less hydrogen.
I remember when I started organic chemistry 1, we completely dropped Lewis structures by week 2! So getting this stuff very quick is important or organic chemist's drawings look like hieroglyphs.
-
Yes, they are the same thing.
Click on the link to see examples of bond line notation.
-
Answer:
Bond line notation is a shorthand way of writing organic structural formulas:
Explanation:
- The carbon atoms and the hydrogen atoms attached to them are not shown.
- Only the bonds between the carbon atoms are shown as lines.
- The vertices and end of lines represent the carbon atoms.
- Any unfilled valences on carbon are assumed to be filled by hydrogen atoms.
- All atoms other than carbon, plus any hydrogen atoms attached to them, are shown.
For example, the bond line notation for propane is

In the above diagram, the vertex and the ends of the lines represent the carbon atoms.
The two end C atoms each contain three H atoms, since they have each formed one bond to the middle C atom.
The middle C contains only two H atoms, since it has already formed two bonds with the other carbons.
Some more examples are shown below.
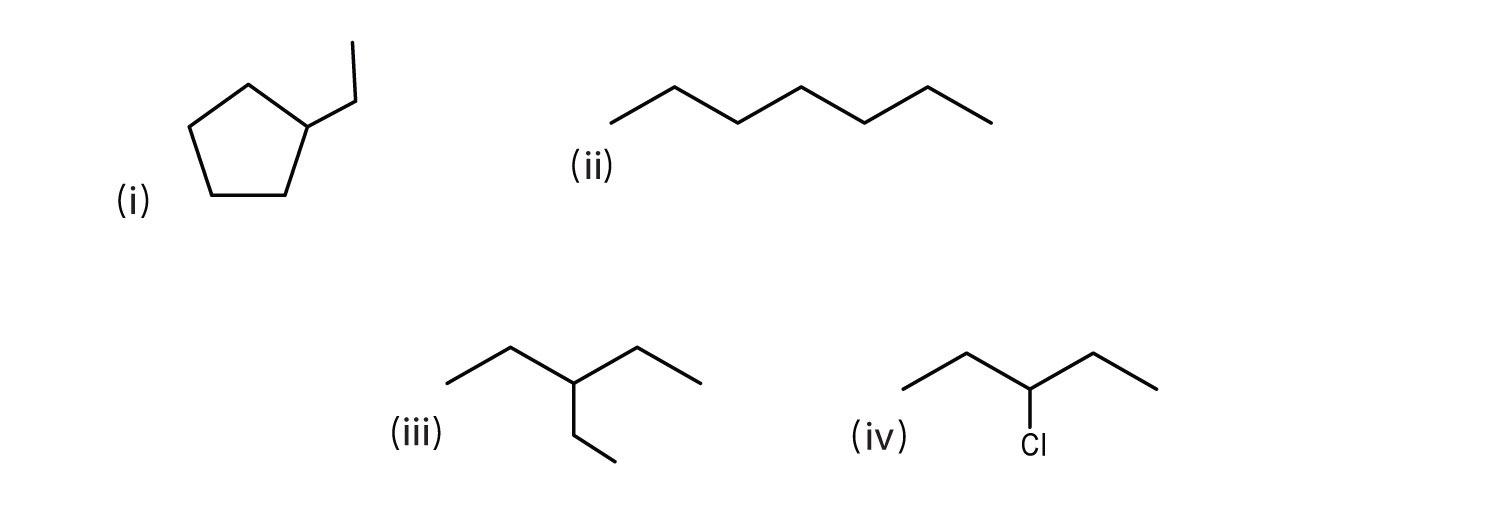
(i) ethylcyclopentane
(ii) heptane
(iii) 3-ethylpentane
(iv) 3-chloropentaneHere are still more bond line structures.

Note that we must show the H atoms that are attached to O and N atoms.
Here’s a video on bond line notation.