Question #33d8c
1 Answer
Oct 14, 2015
The answer is (c)
Explanation:
Each electron shell corresponds to a different energy level, which is represented by the principal quantum number,
The
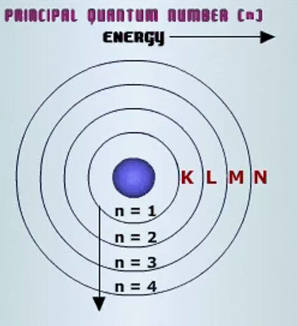
The relationship between the principal quantum number and the number of orbitals is given by the equation
#"no. of orbitals" = n^2#
This equation will produce
#n = 1 implies "no. of orbitals" = 1^2 = 1#
#n = 2 implies "no. of orbitals" = 2^2 = 4#
#n=3 implies "no. of orbitals" = 3^2 = 9#
The
- one 3s-orbital
- three 3p-orbitals
- five 3d-orbitals

