Question #33d8c
1 Answer
Oct 14, 2015
The answer is (c)
Explanation:
Each electron shell corresponds to a different energy level, which is represented by the principal quantum number,
The
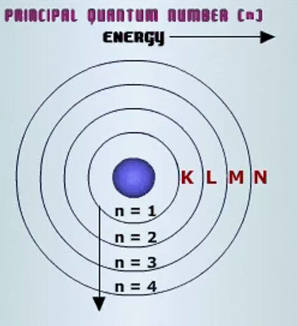
The relationship between the principal quantum number and the number of orbitals is given by the equation
"no. of orbitals" = n^2
This equation will produce
n = 1 implies "no. of orbitals" = 1^2 = 1
n = 2 implies "no. of orbitals" = 2^2 = 4
n=3 implies "no. of orbitals" = 3^2 = 9
The
- one 3s-orbital
- three 3p-orbitals
- five 3d-orbitals

