What are #(1)# the reaction mechanism for 1-hexene reacting with #"H"_2"O"# with catalytic #"H"_2"SO"_4# and #(2)# the reaction of cyclopentene with #"H"_2# in the presence of #"Ni"#?
2 Answers
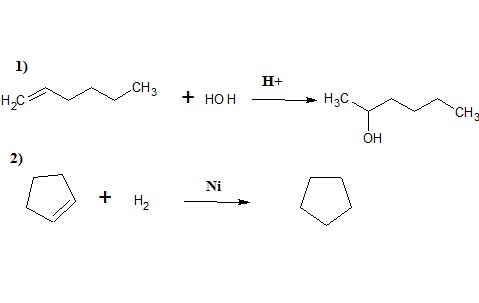
1) This is hydration reaction of alkene, Here addition of water molecule occurs following Markownikoff's rule.(see the link
https://en.wikipedia.org/wiki/Markovnikov's_rule)
2) This is catalytic hydrogenation of a cycloalkene
1) This reaction is an acid-catalyzed hydration as follows:
What we have going on is that:
- The strong-acid solution, presented as
#"H"^(+)# for shorthand, contains protonated water (i.e.#"H"_3"O"^(+)# ). The hydronium protonates the alkene to generate a carbocation intermediate. - The major intermediate that forms is a secondary (
#2^@# ) carbocation, which is more stable than the primary carbocation that also somewhat forms. You should review the effects of hyperconjugation on carbocation stability to see why it is that this occurs. The result is what is called a Markovnikov addition. - Water can act as a strong-enough nucleophile in this scenario, and add onto the carbocation intermediate and form a protonated secondary alcohol.
- Finally, the alcohol is deprotonated by water, given that the pKa of a protonated alcohol is about
#-2.4# , while the pKa of hydronium is about#9.4# (thus the equilibrium favors water, the weaker acid, being protonated).
Thus, the final major product is 2-hexanol.
2) Writing nickel implies the usage of Raney Nickel, a nickel-aluminum-alloy catalyst for hydrogenation. This is analogous to catalysis via palladium over carbon (
If we take nickel to be on the
-
#"H"_2# approaches nickel and overlaps its antibonding "group orbital" (#sigma^"*"# ) sidelong with the#3d_(xz)# orbital of nickel. -
Nickel donates
#pi# -electron density from its#3d_(xz)# orbital into this antibonding orbital, weakening the#"H"_2# bond by increasing the antibonding character (thus decreasing the bond order, or the bond strength). This eventually cleaves the#"H"-"H"# bond.
This is done such that
You are not required to know the mechanism for this, but you should remember that the result is a syn addition hydrogenation.
Thus, the product is cyclopentane, i.e. the reactant cycloalkene with no double bond.


