"TeCl"_2: The central "Te" atom has 6 valence electrons. When the two "Cl" atoms bond to the central atom, they each contribute one electron to form two sigma bonds. This leaves 4 electrons on the central atoms in the form of 2 lone electron pairs.
This is the VSEPR shape "AX"_2"E"_2, a tetrahedron shape with two electron pairs and two atoms, creating a bent molecular shape.
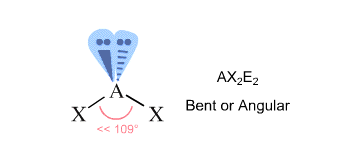 www.chemthes.com
www.chemthes.com
"BeCl"_2, on the other hand, has "Be" as its central atom. Since "Be" only has 2 valence electrons, the two sigma bonds formed by the "Cl" atoms leave "Be" with no lone pairs.
The VSEPR shape is "AX"_2"E"_0 and forms a linear shape.
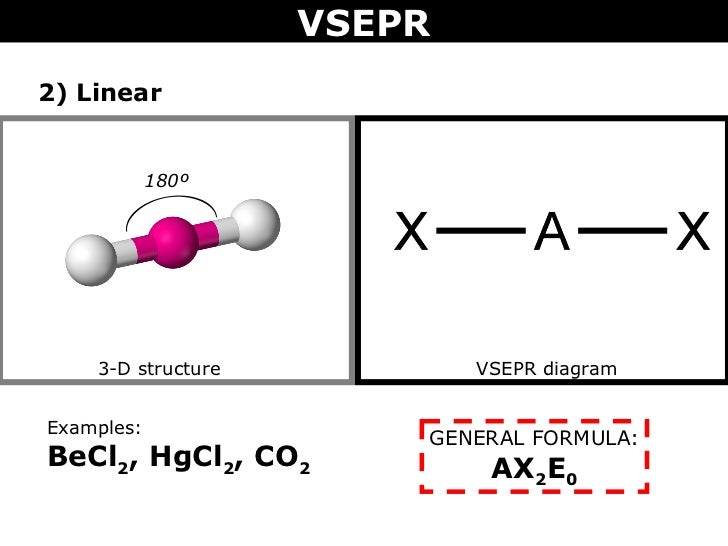 image.slidesharecdn.com
image.slidesharecdn.com
![]() www.chemthes.com
www.chemthes.com image.slidesharecdn.com
image.slidesharecdn.com
