Molecular Geometry
Key Questions
-
Answer:
Molecular geometry is used to determine the shapes of molecules.
Explanation:
The shape of a molecule helps to determine its properties.
For example, carbon dioxide is a linear molecule. This means that
#CO_2# molecules are non-polar and will not be very soluble in water (a polar solvent).Other molecules have different shapes. Water molecules have a bent structure. This is one reason why water molecules are polar and have properties such as cohesion, surface tension and hydrogen bonding.
This video discusses the basics of VSEPR theory which is used to determine the shapes of molecules.
Understanding molecular geometry also helps scientist to understand the shapes of more complex molecules such as proteins and DNA. The shapes of these molecules play incredibly important roles in determining the jobs performed by these molecules in our bodies.
-
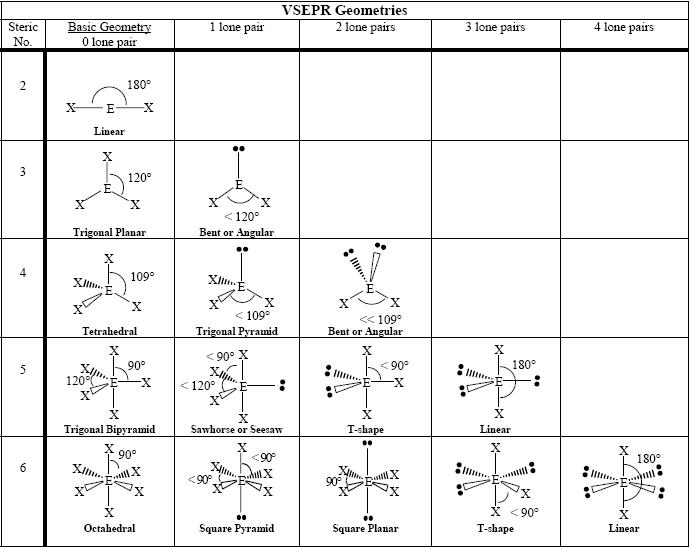
Find the central molecules' steric numbers.
This is the total number of electron pairs and bonds with other atoms.
Take, for example, ammonia:
#N# , the central atom, has a steric number of#4# , calculated by the#3# atoms it's bonding with#+1# lone pair.Thus, it is in the form
#AX_3E_1# , which forms a trigonal pyramidal shape.Consider
#SOF_4# .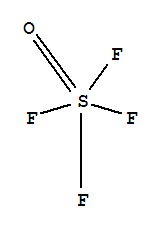
Its steric number is
#5# due to the#5# bonded atoms to the central#S# atom plus#0# lone electron pairs. As it has a VSEPR shape#AX_5E_0# it is a trigonal bipyramid.An easy way to remember all the shapes:
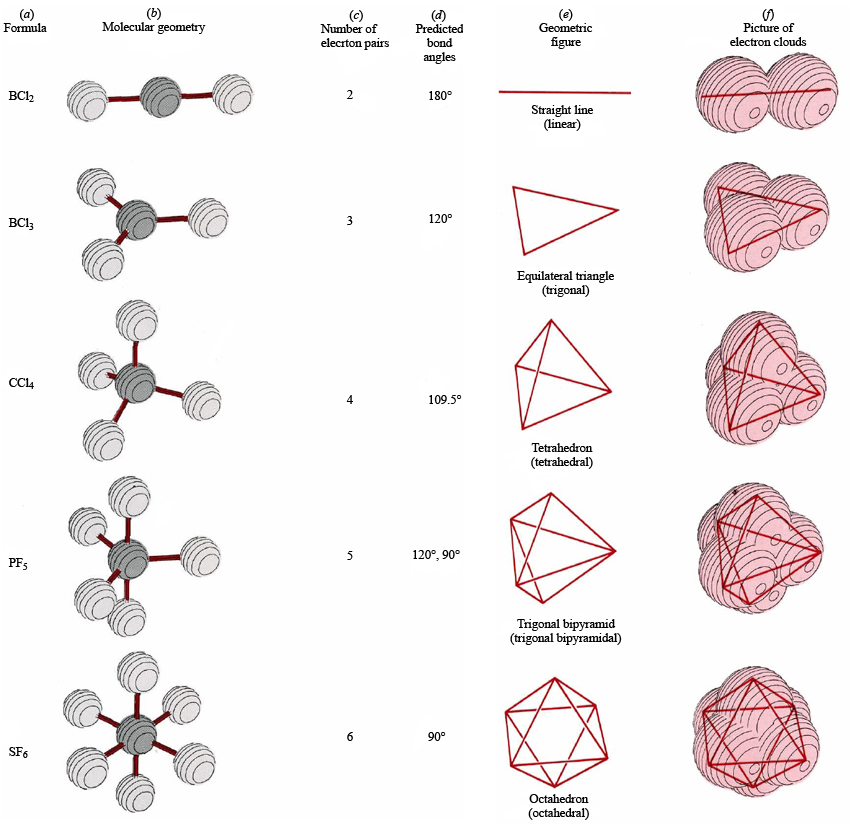
Each steric number has a same "basic shape":
#2# : line
#3# : trigonal plane (a flat equilateral-triangle-looking shape)
#4# : tetrahedron (a triangular pyramid)
#5# : trigonal bipyramid (a trigonal planar shape with two "spokes")
#6# : octahedron (a flat square with two "spokes")Once there are any electron pairs, one spoke of the original shape gets "eaten up": for example, a
#AX_4E_2# is an octahedron shape, but the two "spokes" are taken up by electron pairs, so you're left with just the square—a square planar shape.Similar logic applies to all the shapes, you just have to remember which "spoke" will be taken up by an electron pair.
-
Molecular geometry is a way of describing the shapes of molecules. It applies a theory called VESPR for short.
VESPR stands for valence shell electron pair repulsion. This theory basically says that bonding and non-bonding electron pairs of the central atom in a molecule will repel (push away from) each other in three dimensional space and this gives the molecules their shape.
We can use the following notations when examining a Lewis structure of a molecule.
A = central atom
X = peripheral atoms
E = non-bonding electron pairs of the central atom#AX_2# = linear molecule
#AX_3# = trigonal planar
#AX_3E# = trigonal pyrimidal
#AX_2E_2# = bent
#AX_4# = tetrahedral moleculeHere are some examples:
#H_2O# we need to consider the central atom of water which is oxygen. The oxygen has two bonding electron pairs (single bond to each H) and two non-bonding pairs giving water a#AX_2E_2# conformation and a bent shape.#CO_2# is#AX_2# = linear molecule
#BH_3# is#AX_3# = trigonal planar
#NH_3# is#AX_3E# = trigonal pyrimidal
#CH_4# is#AX_4# = tetrahedral molecule