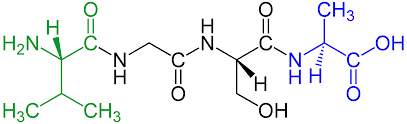
How are peptide bond formed?
1 Answer
Apr 4, 2016
Through condensation reactions, where water is released and one amino acid bonds to another.
Explanation:
Amino acids have the basic structure of
They look like this:
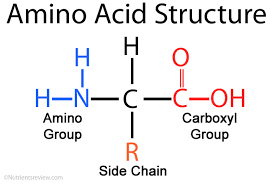
When peptide bonds form, between one amino acid and another, the hydroxyl (
Carboxylic acid groups bond to amino groups, and vice versa.
The hydroxyl group and hydrogen combine to form water (
An amino acid polymer or polypeptide looks like this: