How do you name alkenes with two double bonds?
1 Answer
You name them like ordinary alkenes, but with the ending -diene.
See How do you name alkenes with double bonds?
Here are some more examples.
EXAMPLE 1
Give a name for

Solution
We have a six-carbon chain with two double bonds, so this is a hexadiene.
The double bonds start at C-1 and C-5, so this is a hexa-1,5-diene.
There is a bromine atom at C-3 and a methyl group at C-5. So this is 3-bromo-5-methyl-1,5-methylhexa-1,5-diene.
EXAMPLE 2
Give a name for
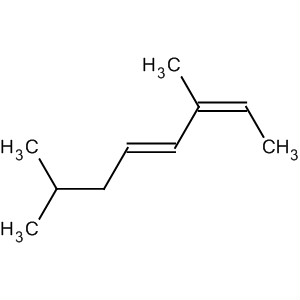
Solution
We have an eight-carbon chain with two double bonds, so this is an octadiene.
The double bonds start at C-2 and C-4, so this is an octa-2,4-diene.
There are methyl groups at C-3 and C-7. So the name without stereochemistry is 3,7-dimethylocta-2,4-diene.
The two low-priority groups on the first double bond are the H on C-2 and the CH₃ at C-3. They are on the same side of the double bond, so the configuration is
The two low-priority groups on the second double bond are the H atoms on C-3 and C-4. They are on opposite sides of the double bond, so the configuration is
The full name, including stereochemistry, is (

