What is a Grignard reagent used for?
2 Answers
A Grignard reagent is used for the making of
Explanation:
While there are a wealth of organic reactions, the number of
The Grignard reagent will react with ketones and aldehydes to form tertiary and secondary alcohols; with ethylene oxide to a primary alcohol that is 2 carbons longer; and with
Still the best way to make a carboxylic acid is to pour an ethereal solution of the Grignard onto dry ice (and stand back). A magnesium carboxylate salt is formed that gives the carboxylic acid after work up:
The Grignard reagent is not particularly effective in making
Grignard
Explanation:
hi Grignard reagent is used to increase the carbon chain
as Grignard look as
RMgX
i.e R here is negatively charged as carbon cannot hold that negative charge
it has to attack
and act as nucleophile
1.How dose it works
as it is negatively charged it attcks on positive charged species or partially positively charged species
i.e! carbon with partially positive charged
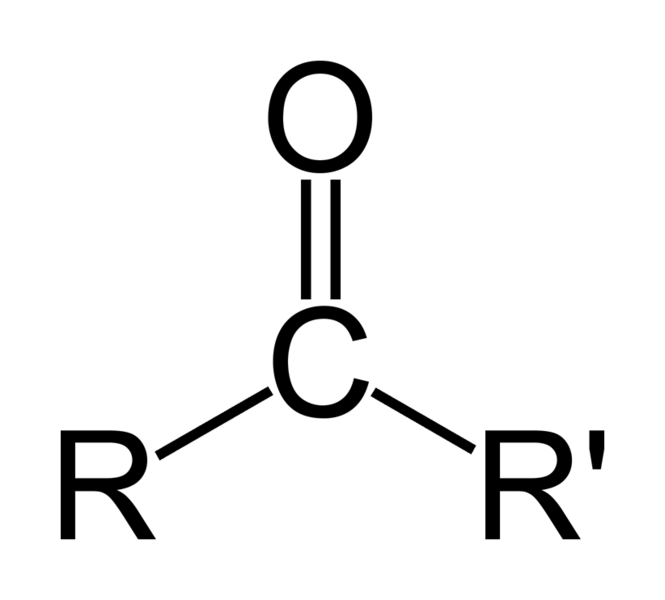
As oxygen is Electronegative atom it pulls electron towards itself and hence become partially negatively charged
and carbon becomes partially positively charged specie
Now grignard with negative carbon attacks on this
oxygen pulls the bond as it is ok with negative charge on it
this negative charge is balanced by hydrolysis
this much is enough
But is carbon has some another strong leaving group besides this double bond and adjacent carbon like this 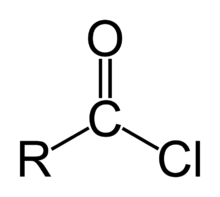
as here it is chlorine
Grignard attcks
As when Chlorine is present Partial positive exists on carbon
and hence grignard attcks
on this attck
1.as oXygen didnt mind to take negative on it as it is electronegative atom
chlorine is so
and hence in attck of grignard ocurs two time BUT HERE OH IS NOT FORMED
WHY ??????
AS when O negative is formed in above step we Hydrolysed it But Now as soon as O NEGATIVE is formed Bond sHIfts down
and c double bond O is formed again and we get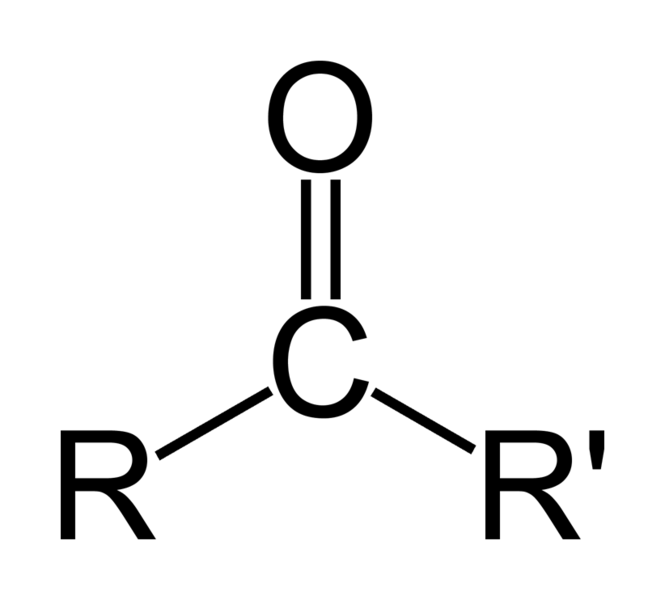
As cholrine leaves
And hen we need 2 moles of grignard
as one for the formation of O negative and other when chlorine leaves
as the strong leaving group around increase moles is increased
PLS NOTE
1. GRIGNARD REACTION IS CARRIED OUT IN DRY ETHER
2. AS SOME IF NOT
R negative will react with solvent and the product which we need is not obtained effectively


