PBr_5's molecular geometry is trigonal bipyramidal.
Let's start with its Lewis structure. P has 5 valence electrons and Br has 7 valence electrons, which means that a total of 5 + 5*7 = 40 electrons must be accounted for.
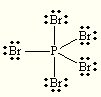 http://www.chem.purdue.edu/vsepr/prelab_answers.html
http://www.chem.purdue.edu/vsepr/prelab_answers.html
P's bonds with the five Br atoms account for 5 * 2 = 10 electrons, while the three lone pairs on each Br atom complete the rest of the electrons - 5 * 6 =30 electrons.
SInce P forms bonds with 5 other atoms and has no lone pairs, it has a steric number and a coordination number equal to 5 . According to VSEPR Theory, this corresponds to a trigonal bipyramidal molecular geometry.
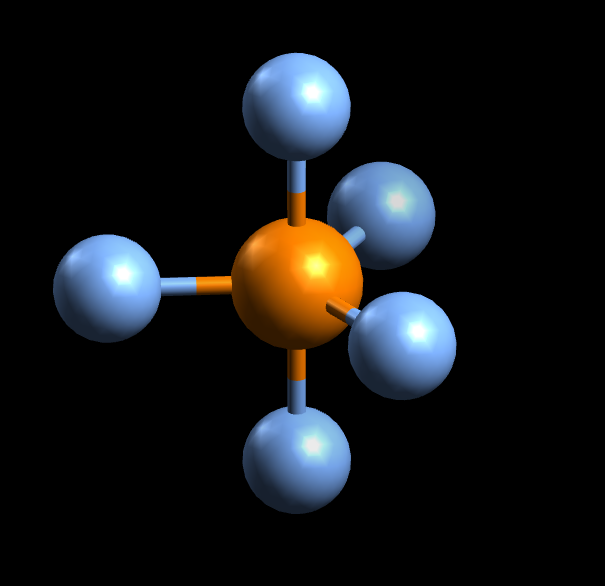 http://chemistry.stackexchange.com/questions/16992/asymmetry-in-trigonal-bipyramidal-geometry
http://chemistry.stackexchange.com/questions/16992/asymmetry-in-trigonal-bipyramidal-geometry
 http://www.chem.purdue.edu/vsepr/prelab_answers.html
http://www.chem.purdue.edu/vsepr/prelab_answers.html  http://chemistry.stackexchange.com/questions/16992/asymmetry-in-trigonal-bipyramidal-geometry
http://chemistry.stackexchange.com/questions/16992/asymmetry-in-trigonal-bipyramidal-geometry 
