Which molecules will undergo aromatic bromination (#"Br"_2, "FeBr"_3#) the fastest? Why?
- phenol
- anisole
- diphenyl ether
- acetanilide
- 4-bromophenol?
- phenol
- anisole
- diphenyl ether
- acetanilide
- 4-bromophenol?
2 Answers
Mar 18, 2016
Phenol
Explanation:
The
Mar 20, 2016
Aromatic bromination is an electrophilic aromatic substitution (EAS) reaction, which will require benzene to act as a nucleophile to acquire an electrophile.
Therefore, any directing groups that activate the ring will make it react more quickly with respect to aromatic bromination.
Activating groups are more electron-donating groups (EDGs), and deactivating groups are more electron-withdrawing groups (EWGs).
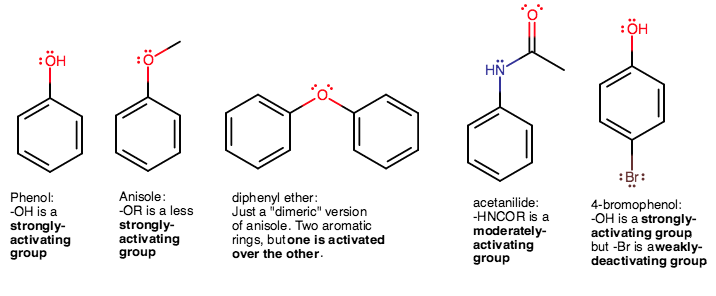
- Phenol, having a good EDG, strongly activates the ring.
- Anisole, having an ether substituent, has hyperconjugation between the oxygen's filled
#pi# orbital electrons and the methyl's#"C"-"H"# #sigma# -bonding electron pair as a opposing effect to the electron donating behavior of#-"OR"# , which weakens the electron-donating capacity of the substituent, giving anisole a less strongly-activated ring. - Diphenyl ether, having only one ether group sharing two aromatic rings, only activates one of the rings. Not a great candidate, and worse than anisole.
- Acetanilide, having a decent EWG, is a moderately-activated ring.
- 4-bromophenol has both an
#-"OH"# and a#-"Br"# on opposite sides.#-"OH"# is a strongly-activating group (high-lying#sigma# MO), but#-"Br"# is a weakly-deactivating group (due to its filled#pi^"*"# , rather than filled#pi# MOs). These, being para to each other, counteract each other and make the ring overall approximately moderately-activated, depending on the extent of deactivation. Either way, not the best.
Therefore, since phenol contains the most strongly-activated ring towards EAS, it participates fastest in EAS.


