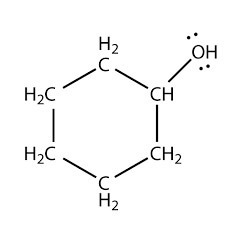
Why is cyclohexanol a secondary alcohol?
1 Answer
Sep 13, 2016
Cyclohexanol is a secondary alcohol because the
Explanation:
Carbon atoms in an organic compound may be classified as primary, secondary or tertiary based on whether they are attached to a single carbon, two carbon atoms or three carbon atoms.
In cyclohexane, all the carbon atoms are secondary.
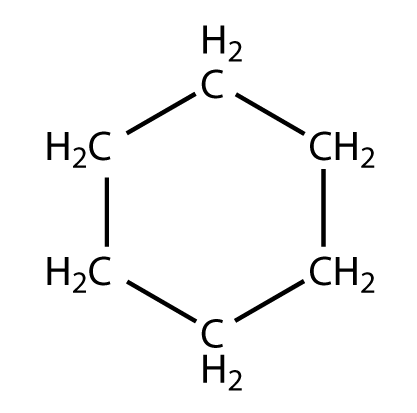
As you may see, all carbon atoms are attached to two carbon atoms on their sides. Thus, all the carbon atoms are secondary.
Thus, when