How do you name branched alkanes?
1 Answer
Here are the rules for naming branched alkanes.
Explanation:
RULES FOR NAMING ALKANES
- Find the longest chain of carbon atoms. This will give you the parent name of the alkane.
- If two chains have the same number of carbons, choose the chain with the most substituents.
- Number the carbons in the chain starting from the end nearest the first substituent.
- If there are substituents that are the same number of carbons in from either end, start the numbering from the end nearest the next substituent.
- When more than one of a given substituent is present, use a multiplying prefix to indicate the number of substituents. Use di- for two, tri- for three, tetra- for four, etc. and use the number assigned to the carbon to indicate the position of each substituent. Use commas to separate numbers from numbers and hyphens to separate numbers from letters. There are no spaces in the final name.
RULES FOR NAMING BRANCHES (ALKYL GROUPS)
- To name an alkyl group, you replace the “-ane” ending of the name of the alkane with the same number of carbon atoms with “-yl”.
- Number the substituent chain starting from the carbon attached to the parent chain (the α carbon).
- If the substituent itself is branched, number the branches starting from the α carbon. From this carbon, count the number of carbons in the longest chain of the substituent. Name the substituent as an alkyl group based on the number of carbons in this chain.
- Place the entire name of the branched substituent in parentheses, preceded by a number indicating the carbon of the parent-chain carbon to which it joins.
- List substituents in alphabetical order. To alphabetize, ignore numerical (di-, tri-, tetra-) prefixes (e.g., ethyl comes before dimethyl), but don't ignore positional prefixes such as iso and neo (e.g., isopropyl comes before neopentyl).
EXAMPLE 1
Name the alkane shown below.
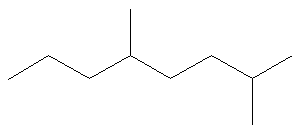
Solution:
1. Choose the longest, most substituted carbon chain. The longest chain is highlighted in red and consists of eight carbons.
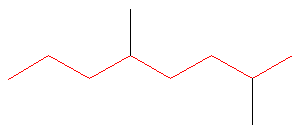
At this point, the parent name is “octane”.
2. Name the substituents. They each contain one carbon atom, so they are both methyl groups. The name now becomes “methyloctane”.
3. Use the multiplying prefix “di-” to indicate that there are two methyl groups. The name is now “dimethyloctane”.
4. Number the parent chain from the end closest to a substituent. If we begin numbering the chain from the right, the groups are numbered 2 and 5. If we begin numbering the chain from the left, the groups are numbered 4 and 7. Therefore, we number the chain from the right as shown below.
The name is now “2,5-dimethyloctane”.
EXAMPLE 2
Name the alkane shown below.
Solution:
1. Choose the longest, most substituted carbon chain. The longest chain contains eleven carbon atoms, so the parent name is “undecane”.
2. Identify the substituents. We have methyl groups at 3 and 5, an ethyl group at 7, and a four-carbon group at 6. The four-carbon group has two carbons (ethyl) as its longest chain, with two methyl groups on the α carbon. The name of the group is therefore 1,1-dimethylethyl. Note: in the name of a substituted substituent, di-, tri-, etc. are part of the name.
3. List the substituents in alphabetical order. The name becomes
“(1,1-dimethylethyl)ethyldimethylundecane”.
4. Insert the locants (the numbers that tell where the substituents are located). The name becomes “6-(1,1-dimethylethyl)-7-ethyl-3,5-dimethylundecane”. Note that a hyphen separates numbers from parentheses. The substituents are in the alphabetical order dimethylethyl, ethyl, methyl.
5. An acceptable but not preferred practice is to name the four-carbon substituent as “tert-butyl”. In that case, another acceptable name would be “6-tert-butyl-7-ethyl-3,5-dimethylundecane”. Here, the substituents are in the order butyl, ethyl, methyl.
Here's a video on the nomenclature of alkanes.


