How do you draw a diastereomer?
1 Answer
You change the configuration of one of the chiral centres.
Explanation:
Diastereomers are stereoisomers that are not superimposable and are not mirror images of each other.
A compound must have at least two chiral centres to have diastereomers.
Let's look at a Compound
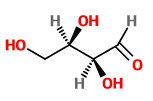
If we change the configuration at
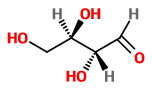
This is not a mirror image of
Now, let's change the configuration of just
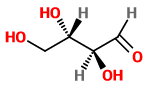
This is also a diastereomer of
BUT, if we change the configuration of both carbon atoms, we get Compound
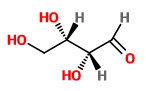
This is nonsuperimposable on
However, it is a mirror image of

