Diastereomers
Key Questions
-
Answer:
Many diastereomers are optically active, but many are not.
Explanation:
By definition a diastereomer is any stereoisomer that is not an enantiomer
Consider the possible optical isomers of 2,3-dichlorobutane.
There are two chiral carbons, so there are
#2^2 = 4# possible optical isomers.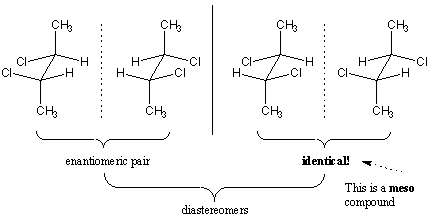
However, two of the structures are identical. They are the same meso compound. So there are only three isomers.
Both of the enantiomers are diastereomers.
In each case, the meso compound is not optically active, while its diastereomeric partner is optically active.
It is even possible to have diastereomeric pairs in which neither member is optically active.
Consider the pentose alcohols, ribitol
and xylitol.
They are diastereomers of each other, but they each have an internal plane of symmetry.
They are both meso compounds, and they are both optically inactive.
-
Answer:
Epimers are always diastereomers.
Explanation:
Diastereomers are compounds that contain two or more chiral centres and are not mirror images of each other.
For example, the aldopentoses each contain three chiral centres.
Thus, D-ribose is a diastereomer of D-arabinose, D-xylose, and D-lyxose.
Epimers are diastereomers that contain more than one chiral center but differ from each other in the absolute configuration at only one chiral center.
Thus, D-ribose and D-arabinose are epimers (and diastereomers), because they differ in configuration only at
#"C-2"# .D-ribose and D-xylose are epimers (and diastereomers), because they differ in configuration only at
#"C-3"# .D-ribose and D-lyxose diastereomers, but they are not epimers, because they differ in configuration at both
#"C-2"# and#"C-3"# . -
Diastereomers are a type of a stereoisomer. Diastereomerism occurs when two or more stereoisomers of a compound have different configurations at one or more of the equivalent stereocenters and are not mirror images of each other.


Also when two diastereoisomers differ from each other at only one stereocenter they are epimers.