1,2,3,4methyl cyclo butadiene is ant aromatic...how??
1 Answer
May 5, 2018
It is anti aromatic.
Explanation:
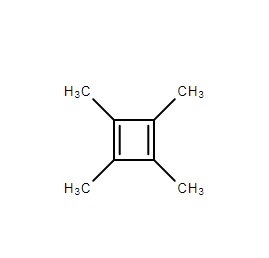
According to the name given by you, the above depicts the compound structure.
Now going by the conditions that qualifies it as an anti aromatic compound
- It is planar. Since you can see the square butadiene structure in the plane
- It forms a conjugative system. Clearly there alternate double bonds and single bond in the given compound.
- It qualifies the rule of having 4n
#pi# electrons in the system to be called as an anti aromatic compound. Since each double bond contributes two#pi# electrons to the system, here you have 2 double bonds and hence 4#pi# electrons.
Above conditions makes it an anti aromatic compound.
Hope it helps!!

