27. Use Hess’ Law to calculate the enthalpy change for the decomposition of dinitrogen pentaoxide?
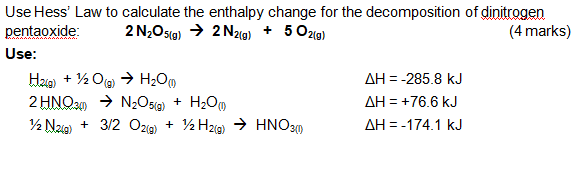
1 Answer
Explanation:
We must construct the overall decomposition reaction from the more elementary reactions.
We need
We need
Now we need to consume the two moles of diatomic hydrogen generated from this last reaction. We can double the first reaction for this.
Now note that all of these reactions add up to the target reaction
sum to
Because enthalpy is a state variable, Hess's Law says the enthalpy change for this reaction is

