3,4-dimethoxybenzaldehyde is treated with bromine.What will be the product/s ?
1 Answer
The product is 2-bromo-4,5-dimethoxybenzaldehyde.
The structure of 3,4-dimethoxybenzaldeyde is
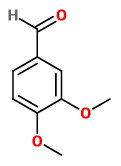 3,4-dimethoxybenzaldeyde
3,4-dimethoxybenzaldeyde
The methoxy groups are activating and ortho, para directing.
The aldehyde group is deactivating and meta directing.
The strongest activators control where the incoming electrophile attacks, so we can consider just the methoxy groups.
The 4-methoxy group will direct to the ortho position
The product will then be 3-bromo-4,5-dimethoxybenzaldehyde.
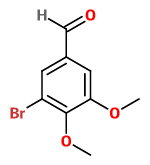 3-bromo-4,5-dimethoxybenzaldehyde
3-bromo-4,5-dimethoxybenzaldehyde
It is possible (but difficult) to prepare aromatic compounds with large substituents on three adjacent carbon atoms (steric effects).
I don’t think this is the correct product.
The 3-methoxy group will direct electrophiles to the ortho position
Attack at
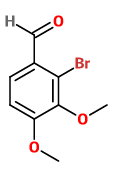 2-bromo-3,4-dimethoxybenzaldehyde
2-bromo-3,4-dimethoxybenzaldehyde
This structure has bulky groups on four adjacent carbon atoms.
If the previous structure with three adjacent groups is unlikely, this one is even less likely.
An attack at
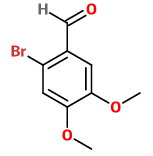 2-bromo-4-5-dimethoxybenzaldehyde
2-bromo-4-5-dimethoxybenzaldehyde
This structure has only two adjacent groups, and steric hindrance is minimized.
I predict that this is the observed product.

