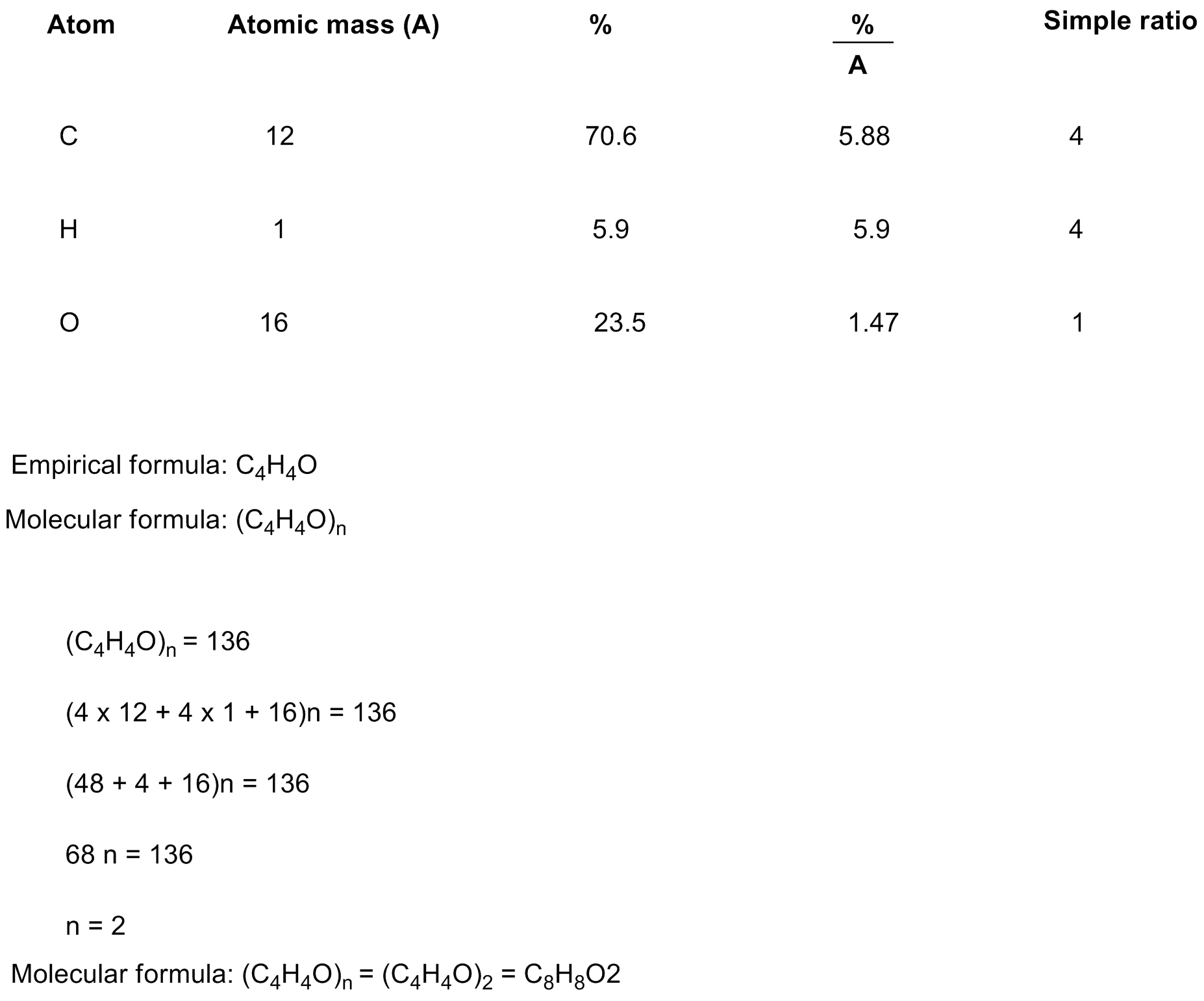
41) A compound that is composed of carbon, hydrogen, and oxygen contains 70.6% C, 5.9% H, and 23.5% O by mass. The molecular weight of the compound is 136 amu. What is the molecular formula? A) B) C) D) E)
A) c8 H8 O2
B)C8 H4 O
C)C4 H4 O
D)C9 H12 O
A) c8 H8 O2
B)C8 H4 O
C)C4 H4 O
D)C9 H12 O
1 Answer
Mar 3, 2018
C8H8O2