Question #d7f4a
1 Answer
Bohr improved Rutherford’s atomic model by proposing that electrons travelled in circular orbits with specific energy levels.
Explanation:
Rutherford proposed that electrons circled the nucleus like planets around the sun.
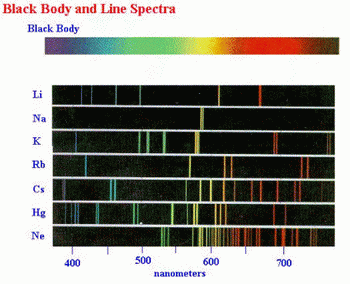
However, his model could not explain atomic line spectra — why metals or their compounds give off characteristic colours when heated.
Bohr improved Rutherford's model by proposing that electrons travelled about the nucleus in orbits that had specific energy levels.
They could jump from one level to another but could not be at any place in between, and they would absorb or emit specific amounts of energy (quanta) when they jumped between levels.
Bohr’s model was an improvement because it explained why the light emitted by atoms consists of lines of certain colours.
When a metal atom is heated, it absorbs energy and the electrons jump to higher energy levels.
When the electrons return to lower levels, they emit this energy in packets of specific energies.
Some of these energies correspond to the colours that we see in the spectra. Others are at energies that our eyes cannot detect.
(from www.askamathematician.com)


