Question #d0227
1 Answer
You look for a plane or an axis of symmetry.
Many students find it difficult to visualize molecules in three dimensions. It often helps to make simple models from coloured sticks and putty or Styrofoam balls.
Planes of Symmetry
A plane of symmetry is an imaginary plane that bisects a molecule into halves that are mirror images of each other.
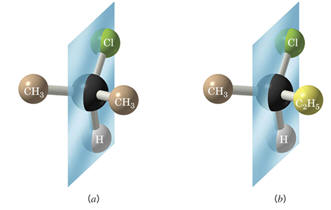
In 2-chloropropane, (a), CH₃CHClCH₃, the vertical plane bisects the H atom, the C atom, and the Cl atom.
The CH₃ group (brown) on the right hand side of the mirror is a mirror image of the CH₃ group (brown) left hand side. So are the left and right halves of the bisected atoms.
 http://isite.lps.org/sputnam/LHS_IB/IBChemistry: mirror image and planes of symmetry in molecules
http://isite.lps.org/sputnam/LHS_IB/IBChemistry: mirror image and planes of symmetry in molecules
So the vertical plane is a plane of symmetry, and the molecule is symmetric.
2-Chlorobutane, (b), CH₃CHClC₂H₅, has a plane that bisects the C, H, and Cl atoms into mirror image halves.
But the C₂H₅ group (yellow) on the right hand side is not a mirror image of the CH₃ group (brown) on the left hand side.
So 2-chlorobutane does not have a plane of symmetry. It has an asymmetric geometry.
Axes of Symmetry
An axis of symmetry corresponds to a rotation by
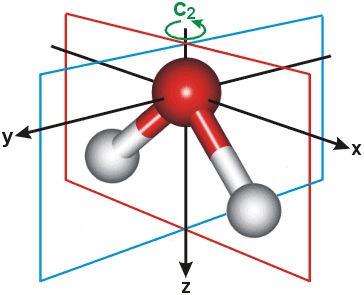
A water molecule has one axis and two planes of symmetry. The water molecule is symmetric.
 tutorvista.com: symmetrical molecule
tutorvista.com: symmetrical molecule
To be symmetric, a molecule must have either an axis of symmetry or an imaginary plane that bisects it into mirror-image halves.
A molecule that has neither of these is asymmetric. CHFClBr has no axes or planes of symmetry, so it is asymmetric.

