How do you do a steam distillation?
1 Answer
You pass steam through an immiscible organic liquid and collect the distillate.
Procedure:
Many organic liquids with high boiling points decompose when you try to distil them. If a liquid X is immiscible with water, you can do a steam distillation instead.
You pass steam through X in a distillation apparatus and collect the distillate. The distillate contains a layer of water and a layer of X, which you can then separate in a separatory funnel.
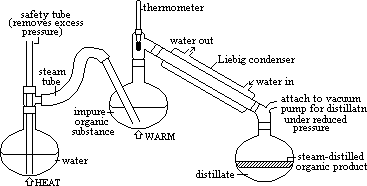
Principle:
Because X is immiscible in water, each liquid exerts its own vapour pressure.
The mixture boils and distils when
Thus, the boiling point of the mixture is less than the boiling point of pure X or pure water. The distillate contains water + X as two immiscible layers.
Example:
The boiling point of bromobenzene is 156 °C, and the boiling point of water is 100 °C.
A mixture of bromobenzene and water boils at 95 °C.
Thus, we can distil bromobenzene at a temperature 61 °C below its normal boiling point.

