Question #d4b59
1 Answer
The dipole moment of 1,4-dihydroxybenzene is not zero because resonance locks the
Explanation:
If 1,4-dihydroxybenzene (IUPAC name benzene-1,4-diol) had the linear structure below, we would predict a dipole moment
If the
With free rotation about the
The dipole moment would be zero.
It appears that the
This prevents free rotation about the
The cis isomer has the
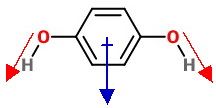
The
The calculated dipole moment of the cis isomer is 2.97 D.
The trans isomer has the
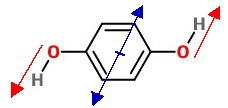
It may look as if the
However, if we slide them to put their origins in the centre of the ring (blue), we see that they are pointing in exactly opposite directions.
The bond dipoles cancel, so the predicted molecular dipole moment is
The observed dipole moment of benzene-1,4-diol is 1.4 D.
It appears that the observed dipole moment is a weighted average of the dipole moments of the two conformations.

