Question #70765
1 Answer
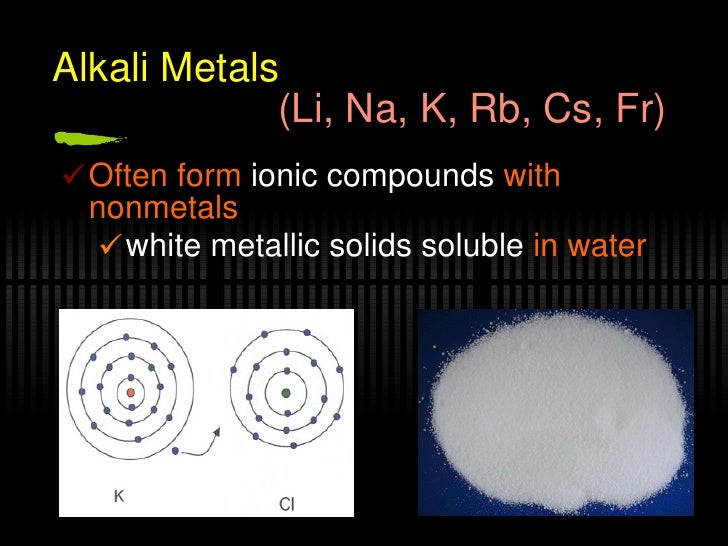
No metals are soluble in water, but almost all the compounds of Group 1 metals are soluble in water.
Most metals do not dissolve in water. Some metals react with water to form hydrogen and a soluble compound of the metal. But this is a reaction (a chemical process) rather than a dissolution (a physical process).
You have probably learned the solubility rule, "All salts of Group 1 metals are soluble". I suspect that your teacher wanted that answer.
The rule is not perfect. Not all salts of Group 1 metals are soluble. For example, many insoluble compounds of sodium exist in nature. These include sodium bismuthate (NaBiO₃), sodium thioplatinate (Na₄Pt₃S₆), and sodium uranate (Na₂UO₄).