How does a covalent bond form?
1 Answer
The sharing of valence electrons produces a covalent bond . Covalent bonds form primarily between nonmetals, and the purpose of the bond is for the atoms involved to become stable, which usually means having eight valence electrons, which known as an octet. Hydrogen is an exception, as it is stable with two valence electrons, known as a duet. The water molecule is a good example to demonstrate covalent bonding.
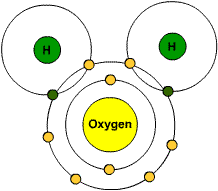
The covalent bonds are the two areas that are overlapping, and you can clearly see by the colors, that each covalent bond is composed of one shared hydrogen electron and one shared oxygen electron. Also notice that each hydrogen atom now has two valence electrons, and the oxygen atom has eight valence electrons (in the outer shell).


