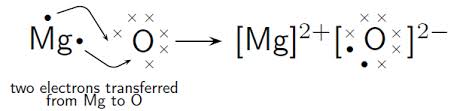Can an atom lose more than one electron to another atom in ionic bonding?
1 Answer
Yes. Atoms can lose more than one electron to another atom in ionic bonding.
Let's take a look at the ionic compound magnesium oxide
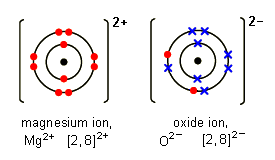
So if the magnesium atom transfers its two valence electrons to the oxygen atom, both will have an octet in their valence shells, and the magnesium atom becomes a magnesium ion with a 2+ charge