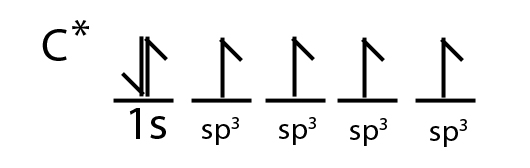What is orbital hybridization?
1 Answer
Sep 8, 2014
Orbital hybridization explains how chemical bonds are able to occur even when the electron configuration of an element indicates that those bonds should not form. For example, according to valence bond theory, carbon can only form two covalent bonds, because of its orbital configuration, in which only two electrons are unpaired.
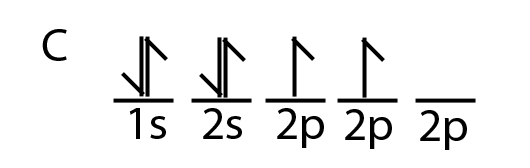
However, we know that carbon forms four covalent bonds. This is possible, because one of the 2s electrons moves to the empty 2p orbital.
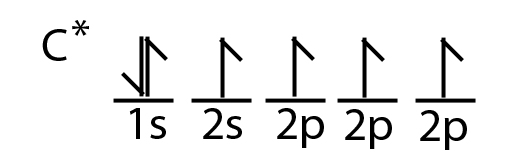
Experiments show that all four covalent bonds formed by carbon have equal energy, which would not be possible if the bonds involved both 2s and 2p orbitals. So the explanation is that the 2s and 2p orbitals hybridize into