Question #fc03a
1 Answer
There are many contributors, so you have to do them systematically.
Vanillin is 4-hydroxy-3-methoxybenzaldehyde. Its structure is

You must consider
1. The two Kekulé structures.
2. Contributors formed by interaction with the carbonyl group.
3. Contributors formed by interaction with the methoxy group.
4. Contributors formed by interaction with the phenolic OH group.
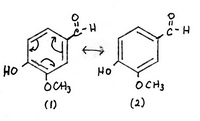
1. The two Kekulé structures
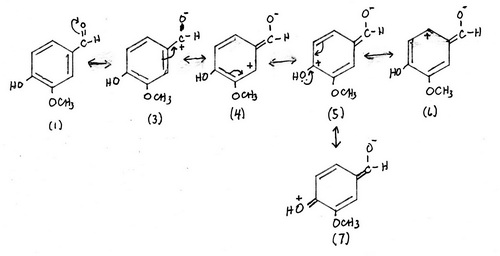
2. Contributors formed by interaction with the carbonyl group
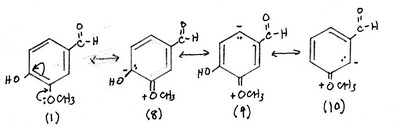
3. Contributors formed by interaction with the methoxy group
4. Contributors formed by interaction with the phenolic OH group
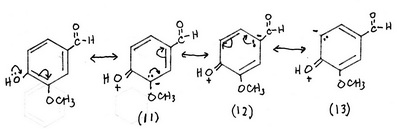
Structures (1) and (2) are the major contributors — there is no charge separation.
Structures (3) to (13) are less important but significant contributors.
The resonance hybrid is shown below.
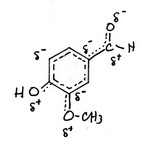
To some extent, the positive charges in structures (4), (5), and (6) cancel the negative charges in structures (10), (9), and (8). The charge distribution in the hybrid is probably as shown above.
Hope this helps.

