Question #e8020
1 Answer
Jul 14, 2014
It increases the surface area of the magnesium and increases the rate of reaction.
Increasing the surface area is one of the factors that affects reaction rates. See
http://socratic.org/questions/what-are-the-four-factors-that-can-affect-a-chemical-reaction-s-rate
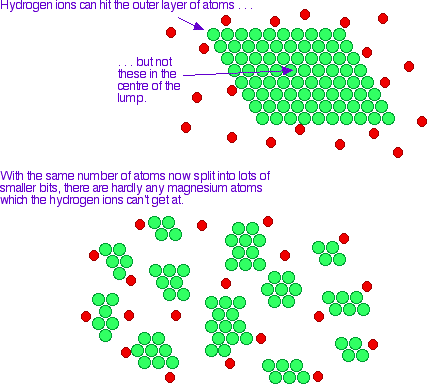
Each cut you make to a strip of magnesium exposes more surface area of magnesium.

If the Mg is reacting with H⁺ ions, for example, more Mg atoms will be exposed to attack. The reaction will be faster.
Dust explosions occur in grain mills because the fine flour dust has such a large surface area. Any spark can cause a fire that quickly becomes an explosion.

