Question #1c5e3
1 Answer
Jul 18, 2014
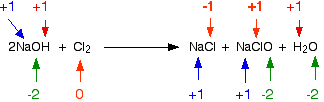
The Cl in ClO⁻ is both oxidized and reduced.
The reaction is a disproportionation — a redox reaction in which a species is simultaneously reduced and oxidized to form two different products.
If you break the reaction into half-reactions, you get
ClO⁻ + 2OH⁻→ ClO₂⁻ + H₂O + 2e⁻
2ClO⁻ + 2H₂O + 2e⁻→ Cl₂ + 4OH⁻
The overall equation is
3ClO⁻ + H₂O → ClO₂⁻ + Cl₂ + 2OH⁻
This shows that a Cl atom in one of the ClO⁻ ions is oxidized to ClO₂⁻, while the Cl atoms in two of the ClO⁻ ions are reduced to Cl₂.
Here's another example of a reaction in which Cl atoms are simultaneously oxidized and reduced.