Question #ef6e6
1 Answer
The atoms of the elements in the alkali metals group, or Group 1/IA on the periodic table have one valence electron. You can tell by working out the electron configurations of the Group 1 elements, which will end with one electron in the outermost s sublevel.
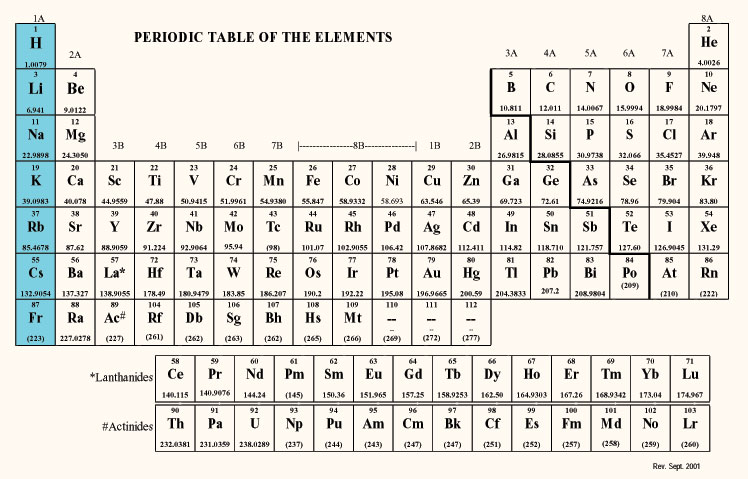
To determine how many electrons are in a neutral atom of a given element, look at the atomic number on the periodic table for that element. The atomic number is the number of protons, which have a positive charge. Electrons have a negative charge, so in a neutral atom, the number of protons (atomic number) is equal to the number of electrons.
The first element in Group 1 is hydrogen (H), which has an atomic number of 1. So a neutral hydrogen atom has one proton and one electron. The electron configuration for hydrogen is
The second element in Group 1 is lithium (Li) with an atomic number of 3. So a neutral lithium atom has 3 protons and 3 electrons. So the electron configuration for lithium is
Sodium (Na) is the third element in Group 1, and it has an atomic number of 11, so a neutral sodium atom has 11 protons and 11 electrons. Its electron configuration is
The National Institute of Standards and Technology in the U.S. has a printable periodic table that provides the electron configurations of all of the elements. You can get it here: http://www.nist.gov/pml/data/periodic.cfm


