What is a single replacement reaction?
1 Answer
In a single replacement reaction, one element is replaced by another in a compound. The general reaction is A + BC
Halogens can also replace other halogens (group 17/VIIA). The general equation is A + XY
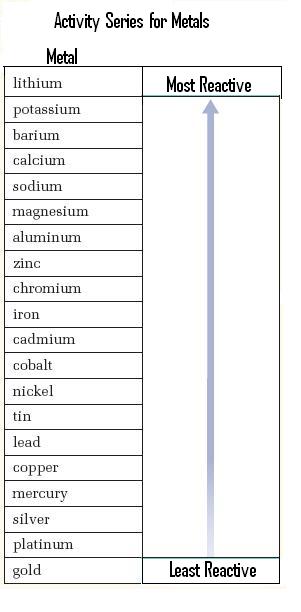
Example 1. Single Replacement of a Metal
Because Fe is above Cu in the activity series of the metals, it is more reactive than Cu, and will replace it in a compound.
The reverse reaction will not occur, because Cu is below Fe in the reactivity series and is therefore less reactive.
Example 2. Single Replacement of a Halogen
Because Cl is above Br in the halogen group, it is more reactive than Br, and will replace Br in the compound.
The reverse reaction will not occur, because Br is below Cl in the halogen group and is therefore less reactive.


