Question #44b23
1 Answer
Here's what I get.
Explanation:
The structural formula is:
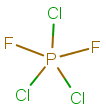
When you draw the Lewis structure for
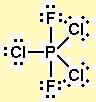
According to VSEPR theory, it must have a trigonal bipyramidal shape.
There are three possible structures:
a) Both
b) Both
c) One
Which is most likely to be correct?
The
- The
#"Cl"# atoms are bigger than#"F"# atoms, so they are more likely to occupy the equatorial locations (separated by 120° rather than 90°). - The
#"P-Cl"# σ bonds occupy more space than the#"P-F"# σ bonds because the less electronegative#"Cl"# atoms hold the electrons less tightly. The#"Cl"# atoms will occupy equatorial locations to minimize bond repulsions.
The structure is most likely.
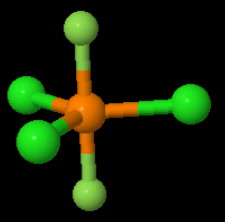
The ball-and-stick model doesn't show correctly the relative sizes of the atoms.
Here's a space-filling picture showing the relative sizes.
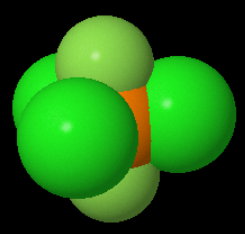
An experiment shows that
The structure shown has no dipole moment:
The two
Thus, the above structure is correct.


