Question #09131
1 Answer
It depends on how much water you evaporate.
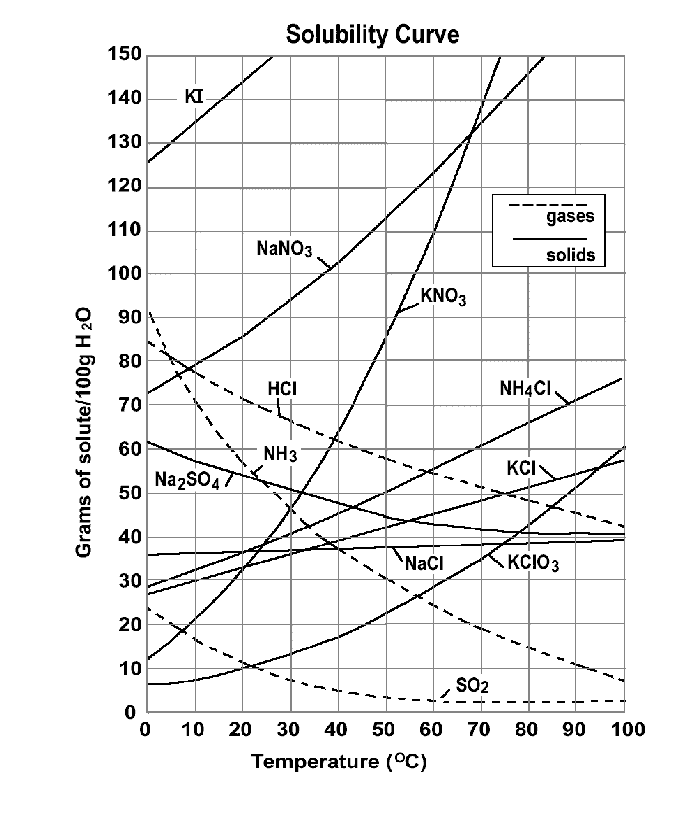
To answer your question, let's look at the solubility curves. Your solution is a mixture of Na⁺, K⁺, Cl⁻, and I⁻ ions.
 www.mrbigler.com
www.mrbigler.com
Between 0 °C and 100 °C, the solubilities of NaCl and KCl are between 27 g and 58 g per 100 g of water.
The solubility of KI never gets below 126 g/100 g of water. The solubility of NaI never gets below 159 g/100 g (it isn't even on the graph!).
Assume that you dissolve 39 g of NaCl and 39 g of KI in 100 g of boiling water.
The solution is saturated with NaCl, but not with KI.
By the time you have cooled the solution to 40 °C, about 3 g of pure NaCl will have crystallized out.
By the time you have cooled the solution to 0 °C, you will have precipitated about 4 g of NaCl and 12 g of KCl. KI and NaI will still remain in solution.
You would probably have to evaporate about two-thirds of the water before KI would precipitate.
The last compound to precipitate on further evaporation would be NaI.


