Question #8fc5c
1 Answer
Error is the amount of deviation from accurate values.
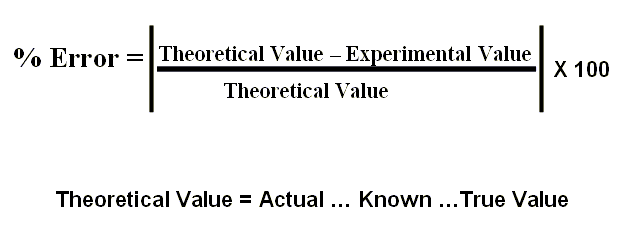
theoretical value is also called actual, known or true value.
Experimental = Values obtained from the lab.
Theoretical = Values obtained from some authority -> book, table, list, official, etc.
(+) A positive percent error indicates your experimental values are lesser than the theoretical values.
(-) A negative percent error indicates your experimental values are greater than the theoretical values.
After heating a 10.00 g sample of potassium chlorate, a student obtains an amount of oxygen calculated to be 3.90 g. Theoretically, there should be 3.92 g of oxygen in this amount of potassium chlorate. What is the percent error in this experiment?
Experimental amount of Oxygen = 3.90g
Theoretical amount of Oxygen = 3.92 g

